Fritextsökning
Artiklar per år
Innehållstyper
-

Oncopeptides läkemedel godkänt i Storbritannien
-

Studie: Apkoppor kan smitta före symtom
Över hälften av dem som fått apkoppor i det pågående utbrottet kan ha smittats av personer som ännu inte haft några symtom av sjukdomen, enligt en brittisk studie.
-
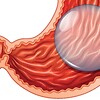
Alert from the Swedish Medicines Agency: Many complications with gastric balloons
According to the Swedish Medicines Agency, an increasing number of serious complications are being reported in procedures with gastric balloons as a method for weight loss. The authority fears significant shortcomings in the information to patients both
-

Marie Gårdmark: Potential step change – EU regulators get to play with data
A new pilot from EMA is starting in September to assess wether the analysis of 'raw data' by regulatory authorities improves the evaluation of marketing approval for new medicines. Marie Grådmark writes in a column that she is looking forward to the outcome of the pilot to hopefully then understand if “in house” analyses actually will add value.
-

Anna Törner: Kalashnikovs in a new guise
Thanks to resisting European regulatory authorities, Europe has been spared the opioid epidemic. In the 1960s, the situation was the opposite as the American pharmaceutical authority, the Food and Drug Administration (FDA), refused to approve thalidomide (Neurosedyn), writes Anna Törner in a column.
-

No demand for new Covid vaccine – “It will probably be discarded”
So far, just under 6 000 doses of the Covid vaccine from Novavax have been used in Sweden, leaving over 1.4 million doses in stock. “They will probably be discarded due to lack of demand in Sweden as well as globally,” says Sweden’s National Vaccine Coordinator Richard Bergström to Life Science Sweden.
-

Det här vet vi om det nya utbrottet av apkoppor
Utbrottet av apkoppor som uppstått väcker förvåning eftersom sjukdomen nu huvudsakligen sprids mellan människor, vilket tidigare varit ovanligt.
-

Det här vet vi om den nya coronavarianten omikron XE
En ny rekombinant variant av coronaviruset med namnet omikron XE har väckt uppmärksamhet. Life Science Sweden frågade Niklas Arnberg, professor i virologi vid Umeå universitet, vad vi vet om den nya varianten.
-

WHO: Nya omikronvarianten XE kan vara mer smittsam
Den nya varianten omikron XE kan vara tio procent mer smittsam än de tidigare omikronvarianterna, rapporterar WHO och uppmanar länder att återuppta sekvensering för att kunna upptäcka nya varianter.
-

The route to vaccines for everyone: “We did not just sit around and wait”
The pandemic was in full swing, and no one knew when or even if a vaccine would come. At that point, the Swedish Minister of Social Affairs called with a proposal, and Richard Bergström did not hesitate. “I already had a notion that this would work,” he says in an interview with Life Science Sweden.
-

Efter positiva resultat för Valneva: ”Ansökan till EMA skickas så snart som möjligt”
Efter att ha stoppats av Storbritannien ser det nu ljusare ut för Valnevas covid-19-vaccinkandidat sedan positiva fas III-resultat presenterats. Nu väntar ansökan till EMA. "Det behövs mer vaccin eftersom hela världen ska få tillgång till vaccin och kunna vaccinera sig", säger Valnevas Sverige-chef Janet Hoogstraate.
-
GMP-expert värvas som utbildningschef på Key2compliance
Magnus Jahnsson har anställts som ny Director of Training & Courses på Key2compliance, ett konsultföretag med fokus på att bistå företag inom life science.
-

Sweden and Denmark – this is how they choose their strategies
Scandinavia’s two major powers in pharmaceutical research have developed strategies for growth in life science, and both countries aim to become world leaders.
-

Marie Gårdmark: Big data evidence generation drives change in the EU regulatory system
Rapid developments in technology have led to the possibility for the generation and analysis of vast volumes of data. Will the availability of big data lead to fundamental changes to the regulatory system? In a column Marie Gårdmark, CEO and Senior Consultant at RegSmart Life Science, develops her views on this subject.
-

Column: Repurposing as a golden ticket to approval
"Hopefully, in the end, patients will be winners by receiving on-label treatments for which benefit-risk has been properly assessed." In a column Marie Gårdmark, CEO at RegSmart Life Science, reflects on drug repurposing.
-

Hemarbete ökar risken för nätfiske i läkemedelsföretag
Allt fler hackerförsök inom läkemedelsbranschen riktar sig mot anställdas mobiltelefoner.
-

Storbritannien godkänner Pfizers covid-19 vaccin
Vaccinet kommer att finnas tillgängligt på brittiska sjukhus från och med början på nästa vecka.
-

Drug development creates new challenges for regulators
-

Krönika: Can regulators keep up with innovation?
New technology has increased the understanding of disease mechanisms and enabled approval of products targeting small but specific patient populations; sometimes referred to as precision medicine. Gene- and cell therapies have reached the market, exemplified by CAR-T cells, and the research pipeline is promising. In addition, the medical device field is constantly growing creating new solutions to address patient needs.
-

Brexit looking back and looking forward
More than 3 years have passed after the vote and there is still uncertainty around the outcome although the UK December 12 election set a clearer course towards an exit early 2020.
-
Meet the governmental life science office
More clinical trials, promotion for digitisation and interoperability together with increased use of data generated by both healthcare and individuals. The duties of the Life Sciences office are plentiful.
-

It´s time to establish relations
Communication with the patient is the key to future healthcare, writes Hanna Brodda, editor in chief of Life Science Sweden.
-
Lucka 4 - vem finns här?
Bakom den fjärde luckan önskar sig vd:n att föreläsaren och succéförfattaren ska slå en signal...
-
They’re all doing it – buying drug research
December 12 the time has come for this year's annual partnering meeting Pharma Outsourcing in Stockholm. In Sweden this is a growing business, with Recipharm as the biggest CRMO of the country.