Fritextsökning
Artiklar per år
Innehållstyper
-

Confidence in childhood vaccines is in decline worldwide
Since the pandemic, confidence in vaccinating children has plummeted. In a new report, UNICEF urges world leaders to act before the situation worsens. In 52 out of 55 countries surveyed, public perception of the importance of vaccinating children has declined.
-

Vinnova is going to establish a new innovation cluster
Vinnova, the Swedish Agency for Innovation Systems, has been commissioned by the government to establish a national innovation cluster for advanced medicines.
-

“An entire industry is about to be wiped out”
According to Jennie Ekbeck, CEO of Umeå Biotech Incubators, Sweden risks not having any small diagnostic companies left in five years.
-

Column: ”Authentic leadership and clear mandates pave the way for more female CEOs”
”I believe that the aspect of having clear mandates and titles on the one hand and women progressing into top positions must be explored further”, Helena Strigård writes in a column.
-

Individual DNA passport could result in fewer drug side effects
You may be required to show a DNA passport when you pick up medicines at the pharmacy in the future. According to a new study, patients might suffer 30% fewer side effects if the drug treatment is adapted to their genes.
-

The government proposes fines for pharmaceutical companies that fail to notify drug shortages in time
According to a compilation from the Swedish Medicines Agency, the number of residually notified medicines increased by 54 % in Sweden last year compared to the previous year. In a bill presented by the government a number of proposals are put forward
-

Scharlakansfeber hos barn väcker oro i Storbritannien
Ett ovanligt stort antal fall av scharlakansfeber har fått Storbritanniens hälsomyndigheter att uppmana föräldrar till uppmärksamhet på sina barns symtom.
-

He is zooming in on topical preparations
According to Zelmic CEO David Sagna, topical products in drug development is a growing market, and to keep pace with the development, the company is awaiting approval for its new GMP facility.
-

Oncopeptides läkemedel godkänt i Storbritannien
-

Studie: Apkoppor kan smitta före symtom
Över hälften av dem som fått apkoppor i det pågående utbrottet kan ha smittats av personer som ännu inte haft några symtom av sjukdomen, enligt en brittisk studie.
-
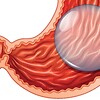
Alert from the Swedish Medicines Agency: Many complications with gastric balloons
According to the Swedish Medicines Agency, an increasing number of serious complications are being reported in procedures with gastric balloons as a method for weight loss. The authority fears significant shortcomings in the information to patients both
-

Marie Gårdmark: Potential step change – EU regulators get to play with data
A new pilot from EMA is starting in September to assess wether the analysis of 'raw data' by regulatory authorities improves the evaluation of marketing approval for new medicines. Marie Grådmark writes in a column that she is looking forward to the outcome of the pilot to hopefully then understand if “in house” analyses actually will add value.
-

Anna Törner: Kalashnikovs in a new guise
Thanks to resisting European regulatory authorities, Europe has been spared the opioid epidemic. In the 1960s, the situation was the opposite as the American pharmaceutical authority, the Food and Drug Administration (FDA), refused to approve thalidomide (Neurosedyn), writes Anna Törner in a column.
-

No demand for new Covid vaccine – “It will probably be discarded”
So far, just under 6 000 doses of the Covid vaccine from Novavax have been used in Sweden, leaving over 1.4 million doses in stock. “They will probably be discarded due to lack of demand in Sweden as well as globally,” says Sweden’s National Vaccine Coordinator Richard Bergström to Life Science Sweden.
-

Anna Törner: To kill your darlings
Hopes were high when Anna Törner and her colleague started a study on a dietary supplement that seemed unbelievably good. “Enthusiastically, we dreamed of exciting results and perhaps a publication in a high-impact journal,” she writes in a column.
-

Det här vet vi om det nya utbrottet av apkoppor
Utbrottet av apkoppor som uppstått väcker förvåning eftersom sjukdomen nu huvudsakligen sprids mellan människor, vilket tidigare varit ovanligt.
-

Det här vet vi om den nya coronavarianten omikron XE
En ny rekombinant variant av coronaviruset med namnet omikron XE har väckt uppmärksamhet. Life Science Sweden frågade Niklas Arnberg, professor i virologi vid Umeå universitet, vad vi vet om den nya varianten.
-

WHO: Nya omikronvarianten XE kan vara mer smittsam
Den nya varianten omikron XE kan vara tio procent mer smittsam än de tidigare omikronvarianterna, rapporterar WHO och uppmanar länder att återuppta sekvensering för att kunna upptäcka nya varianter.
-

The route to vaccines for everyone: “We did not just sit around and wait”
The pandemic was in full swing, and no one knew when or even if a vaccine would come. At that point, the Swedish Minister of Social Affairs called with a proposal, and Richard Bergström did not hesitate. “I already had a notion that this would work,” he says in an interview with Life Science Sweden.
-

Efter positiva resultat för Valneva: ”Ansökan till EMA skickas så snart som möjligt”
Efter att ha stoppats av Storbritannien ser det nu ljusare ut för Valnevas covid-19-vaccinkandidat sedan positiva fas III-resultat presenterats. Nu väntar ansökan till EMA. "Det behövs mer vaccin eftersom hela världen ska få tillgång till vaccin och kunna vaccinera sig", säger Valnevas Sverige-chef Janet Hoogstraate.
-
GMP-expert värvas som utbildningschef på Key2compliance
Magnus Jahnsson har anställts som ny Director of Training & Courses på Key2compliance, ett konsultföretag med fokus på att bistå företag inom life science.
-

Sweden and Denmark – this is how they choose their strategies
Scandinavia’s two major powers in pharmaceutical research have developed strategies for growth in life science, and both countries aim to become world leaders.
-

Marie Gårdmark: Big data evidence generation drives change in the EU regulatory system
Rapid developments in technology have led to the possibility for the generation and analysis of vast volumes of data. Will the availability of big data lead to fundamental changes to the regulatory system? In a column Marie Gårdmark, CEO and Senior Consultant at RegSmart Life Science, develops her views on this subject.
-

Björn Arvidsson: Let's build on the positive momentum
Progress needs narratives and heroes, and more than ever, going into the new year feels like an opportunity for a fresh start.